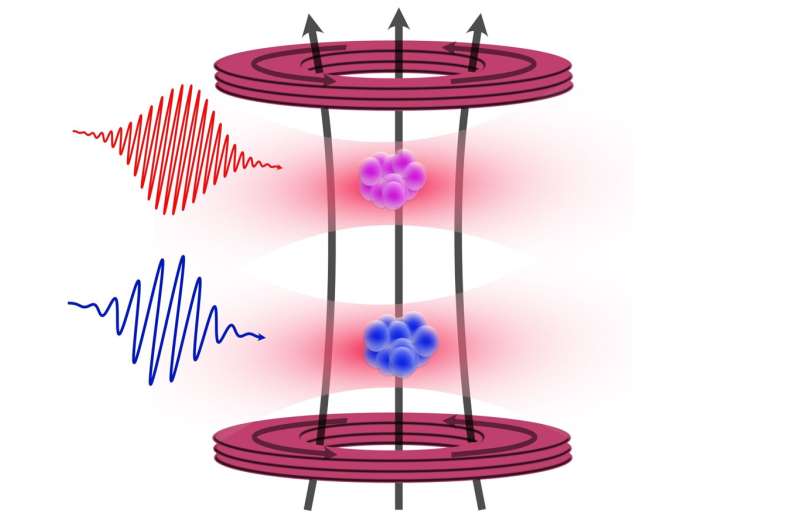
A team of physicists from the University of Amsterdam has achieved a significant breakthrough in measuring the properties of strontium atoms, specifically the 87 Sr isotope, a development that could enhance the precision of atomic clocks and quantum computers. By cleverly utilizing nearby rubidium atoms, researchers have improved the measurement of the nuclear g-factor of strontium by a factor of one hundred, marking a milestone in atomic physics. This research was published in the journal Physical Review Letters this week.
Strontium may not be a household name, but it plays a crucial role in advanced technologies. As one of the alkaline earth metals, strontium has applications in atomic clocks and quantum computing due to its unique nuclear properties. The 87 Sr isotope, with its 38 protons and 49 neutrons, exhibits a special characteristic known as fermionic behavior, which allows its nucleus to function as a tiny magnet. This property is vital for the operation of optical clocks, which rely on the precise emission and absorption of light.
Optical clocks, using 87 Sr, depend on the accurate determination of light frequencies that atoms emit during state transitions. The ideal transition for 87 Sr corresponds to a wavelength of 698 nanometers, offering extreme timing precision. However, bosonic isotopes of strontium cannot undergo this transition due to their zero spin. The nuclear spin of the fermionic 87 Sr allows for the necessary adjustments, making the optical clock transition viable.
Significance of the Zeeman Effect
The research also delves into the Zeeman effect, first discovered by Dutch Nobel laureate Pieter Zeeman in 1896. This phenomenon describes how the energy levels of electrons in an atom split under a magnetic field. For nuclear spins, the Zeeman effect similarly impacts the energy levels of 87 Sr, which can split into up to ten distinct states in the presence of a magnetic field. These states can serve as building blocks for quantum computing, enhancing processing power beyond traditional binary bits.
The team, including first author Premjith Thekkeppatt, initially aimed to create molecules from strontium and rubidium but redirected their focus to the proximity of both elements. By using a technique called optical trapping, the researchers successfully confined 87 Sr and rubidium atoms together without overlap. This arrangement facilitated precise measurements using nuclear magnetic resonance, leading to a better understanding of the g-factor and the Zeeman effect.
Implications for Future Research
The newly determined precision of the g-factor not only advances atomic clock technology but also sets a benchmark for future atomic structure calculations. Thekkeppatt noted that the method developed during this research could inspire similar precision measurements in other atomic species. This breakthrough holds promise for furthering developments in quantum computing and related technologies.
As the field of atomic physics continues to evolve, the implications of this research could pave the way for advancements in both theoretical and practical applications. The enhanced understanding of strontium’s properties positions researchers to explore more sophisticated quantum systems, potentially transforming the landscape of modern computing.
The complete study can be accessed in the Physical Review Letters journal, detailing the methods and results of this significant achievement.