Recent advancements in the production of nitrogen-doped graphene nanoplatelets (N-GNPs) may revolutionize the field of materials science by emphasizing sustainability. A study led by researcher Chamalki Madhusha at Monash University highlights a new solvent-free, bio-derived mechanochemical approach to functionalize graphene, which could reduce environmental impacts associated with traditional methods.
Graphene, often heralded as a “wonder material,” possesses remarkable properties such as exceptional strength, electrical conductivity, and thermal efficiency. Despite this promise, many graphene-based applications have not transitioned successfully from laboratory settings to practical use. One significant hurdle has been the difficulty in dissolving graphene in common solvents, which necessitates complex and environmentally harmful modification processes.
In their recent publication in ACS Sustainable Chemistry & Engineering, Madhusha and colleagues address the challenges associated with traditional functionalization methods. Conventional techniques for nitrogen doping often require toxic nitrogen precursors, extensive purification steps, and high-temperature annealing, all of which contribute to considerable waste and environmental degradation.
Revolutionary Mechanochemical Techniques
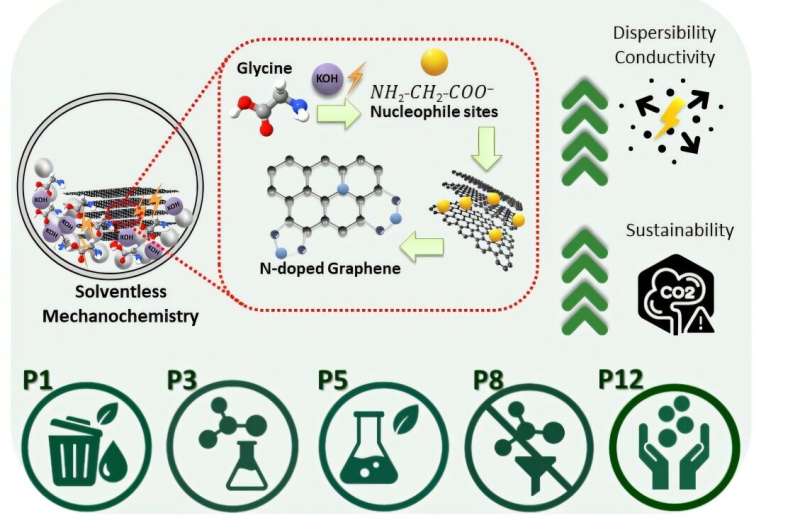
The research team turned to mechanochemistry, a method that utilizes mechanical forces such as shear and friction to drive chemical reactions. This technique has gained traction in green chemistry due to its potential to eliminate solvents and reduce energy demands. By employing a ball-milling process, the researchers successfully functionalized graphite with a bio-derived nitrogen source, specifically amino acids, under ambient conditions.
This innovative approach produced nitrogen-doped graphene nanoplatelets without the need for harmful solvents or toxic reagents. The resulting N-GNPs not only retained high electrical conductivity but also demonstrated improved dispersibility, effectively overcoming two major challenges in graphene processing.
To assess the sustainability of their process, Madhusha and her team evaluated both qualitative and quantitative factors, including waste generation and overall energy consumption. The method achieved an impressive material yield of around 80%, and the E-factor—a standard measure of waste generation—was significantly lower compared to conventional graphene functionalization strategies.
Implications for Advanced Materials
The integration of nitrogen atoms into the graphene lattice enhances the material’s electrical conductivity, chemical reactivity, and interactions with surrounding polymers. The study indicates that N-GNPs can serve as effective nanofillers, capable of improving the mechanical, thermal, and electrical properties of composite systems.
One particularly noteworthy application of N-GNPs is their compatibility with vitrimers—polymers that combine the durability of thermosets with the reprocessability of thermoplastics. Incorporating N-GNPs into vitrimer matrices can lead to electrically triggered self-healing materials, thereby enhancing mechanical strength and conductivity while maintaining the stability of the material’s inherent network.
Beyond the immediate application of graphene, Madhusha’s work carries broader implications for the materials science field. It challenges the reliance on outdated manufacturing processes that do not consider environmental impact. The mechanochemical approach exemplifies how integrating green chemistry principles into materials design can lead to significant reductions in waste and energy usage.
Looking ahead, the research team aims to explore how this sustainable synthesis technique can be adapted for other dopants and composite systems, paving the way for scalable manufacturing solutions. As demand for advanced functional materials rises, sustainable synthesis strategies will be crucial in shaping the technologies of the future.
Madhusha’s findings underscore the importance of aligning nanomaterial innovation with sustainability goals, illustrating that the pursuit of advanced materials need not come at the expense of environmental responsibility.