Geneva-based health technology startup FluoSphera has successfully raised CHF 1.15 million in seed funding to enhance its innovative 3D human organ models, aimed at reducing the high rate of drug failures in clinical trials. This funding round was led by Soulmates Ventures and a Swiss business angel, with additional participation from IndieBio New York.
The development of new medications often spans 10 to 15 years and incurs costs of billions of dollars. Disappointingly, over 90% of drug candidates fail during clinical trials, primarily due to outdated testing methods that do not accurately predict human responses. Traditional cell models lack complexity, and animal testing frequently fails to replicate human reactions, resulting in costly late-stage failures.
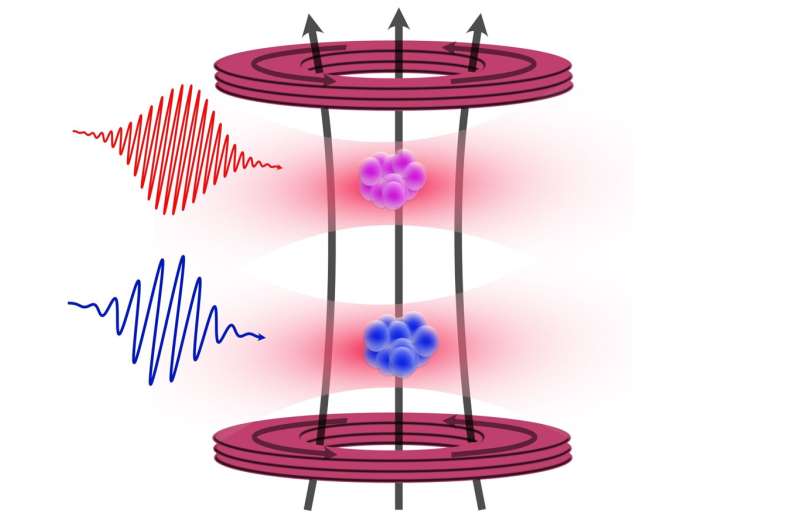
FluoSphera aims to address these challenges through its proprietary 3D multiplexed in vitro platform. Founded in 2021 by Dr. Gregory Segala (CEO/CSO), Dr. Clelia Bourgoint (VP Operations), and Dr. Aurélien Roux (Scientific Advisor), the company enables drug developers to test compounds across multiple human tissue models simultaneously. The platform can integrate up to six or seven different human tissue types in a single well, utilizing fluorescent coding to track each type independently.
This advanced setup allows researchers to observe organ interactions and evaluate both drug efficacy and potential toxicity within a single experiment. By providing earlier and more accurate insights, the platform aids developers in identifying promising molecules sooner while minimizing the risk of late-stage failures.
FluoSphera has already established collaborations with various biotech and pharmaceutical entities, demonstrating notable success in antibody-drug conjugate testing. The company collaborates with Revvity for high-throughput screening and aligns with evolving regulatory frameworks, such as the FDA’s Modernisation Act 3.0, which promotes non-animal testing methods in preclinical research.
According to FluoSphera’s claims, drug developers leveraging this technology could potentially save between $100 million and $500 million per molecule by lowering developmental risks, clinical costs, and time-to-market.
Future Plans and Goals
With the recent funding, FluoSphera plans to scale its commercial collaborations with pharmaceutical companies and contract research organizations (CROs). The capital will also be utilized to strengthen its business development team and enhance its AI and automation capabilities for large-scale imaging analysis. Furthermore, the company intends to expand its presence in both the US and European markets, while also venturing into Asia.
“We’re building the next generation of preclinical tools, not just to expedite the arrival of new medicines to market at a lower cost, but also to enhance the quality of drug discovery processes,” stated Dr. Clélia Bourgoint, CEO and co-founder of FluoSphera. “By improving human relevance and reducing reliance on animal models, we assist our partners in delivering safer, more effective treatments to patients more swiftly.”
Adding to this, Hynek Sochor, Founder and Managing Partner at Soulmates Ventures, expressed confidence in FluoSphera’s potential. “The company stood out to us from the start for its exceptional founders and groundbreaking science. As the pharmaceutical industry transitions away from animal testing, the demand for reliable human-relevant models is immense. FluoSphera is unlocking a multi-billion-dollar opportunity, enabling drug developers to innovate more swiftly, safely, and ethically.”
In conclusion, FluoSphera’s innovative approach to drug testing could significantly reshape the landscape of pharmaceutical development, offering a promising alternative that prioritizes human relevance while reducing costs and the need for animal testing.