A groundbreaking development at the Massachusetts Institute of Technology (MIT) could significantly reduce the cost of mRNA vaccines. Researchers have created a new type of lipid nanoparticle (LNP) that enhances the delivery of mRNA, enabling an effective immune response with just 1/100 of the current vaccine dosage. This advancement not only promises to lower production costs but may also alleviate potential side effects associated with higher dosages.
Daniel Anderson, a professor in MIT’s Department of Chemical Engineering, emphasizes the importance of cost in widespread vaccine distribution. “When you think about the cost of making a vaccine that could be distributed widely, it can really add up,” he stated. The study detailing this innovation has been published in Nature Nanotechnology.
Enhanced Delivery Mechanism
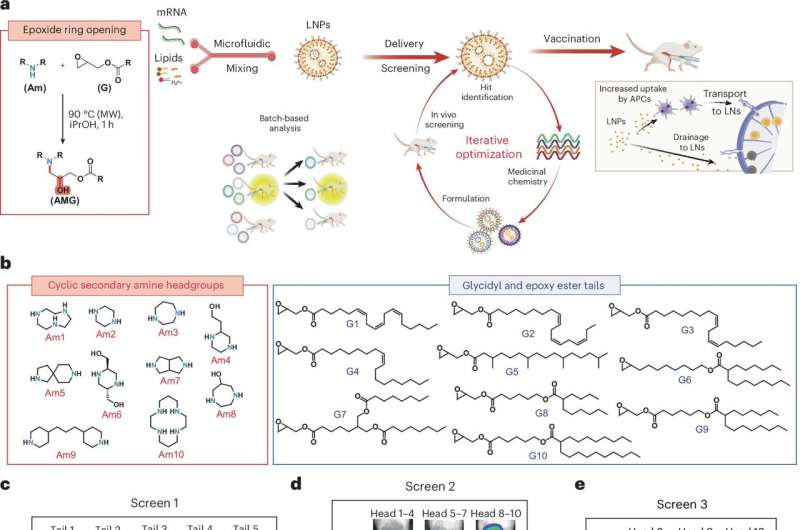
The challenge with mRNA vaccines lies in their stability. To protect these vaccines from degradation after injection, they are encapsulated in lipid nanoparticles. These nanoparticles facilitate the entry of mRNA into cells, allowing it to produce fragments of proteins from pathogens like influenza or SARS-CoV-2. The MIT team focused on developing LNPs that could deliver vaccines at a significantly lower dose without compromising efficacy.
The researchers designed a library of new ionizable lipids, which included cyclic structures that enhance mRNA delivery. By screening various combinations in mice, they identified a particularly effective particle called AMG1541. This innovative LNP demonstrated superior performance in overcoming a significant delivery barrier known as endosomal escape, which is crucial for mRNA’s effectiveness.
Another advantage of AMG1541 is its biodegradability. The ester groups in these nanoparticles allow them to break down after delivering their payload, facilitating quicker removal from the body and potentially reducing side effects.
Promising Results in Vaccine Trials
To test the efficacy of the AMG1541 LNP, researchers administered an mRNA influenza vaccine to mice. They compared its performance against a vaccine made with the FDA-approved lipid SM-102, which was utilized in Moderna’s COVID-19 vaccine. Remarkably, mice vaccinated with AMG1541 generated an equivalent antibody response but required only 1/100 of the dose.
“It’s almost a hundredfold lower dose, but you generate the same amount of antibodies, so that can significantly lower the dose,” explained Arnab Rudra, a visiting scientist at the Koch Institute. This reduction in dosage not only has implications for cost but also for the overall safety profile of the vaccine.
The new LNPs were also found to be more effective at delivering their mRNA to antigen-presenting cells, critical players in the immune response. These cells are responsible for processing foreign antigens and activating other immune cells, such as B and T cells. Furthermore, the new particles showed a higher tendency to accumulate in lymph nodes, where they can interact with a greater number of immune cells.
The flexibility of this technology means it could be adapted for a variety of vaccines, including those for COVID-19 and HIV. “Akash Gupta, a research scientist at the Koch Institute, remarked, “We have found that they work much better than anything that has been reported so far.”
This research opens up exciting possibilities for the future of vaccines. As Kaelan Reed noted, the use of mRNA technology could allow for more timely vaccine production, particularly for flu vaccines, which traditionally require long lead times for manufacturing.
The implications of this study are profound, as they suggest a path toward more affordable and accessible vaccines worldwide. As the researchers continue to explore the potential of their LNPs, the hope is that these innovations will contribute to improved public health outcomes in the face of global infectious diseases.
Further details of this research can be found in the paper titled “Degradable cyclic amino alcohol ionizable lipids as vectors for potent influenza mRNA vaccines,” published in Nature Nanotechnology on November 8, 2025.